Abstrict
This review focuses on the physicochemical properties of nanocarriers (NCs) and the physical properties of the tumor microenvironment (TME), highlighting strategies used to specifically deliver molecules of interest to specific lesions. This review discusses these products, explains the simple selection of high-performance and active methods, and illustrates this with examples of drugs that are focused on clinical research or treatment. The last few years have seen an increase in targeted delivery of anticancer drugs. Although many trials have been completed, only a few targets of nanocarriers have been approved for clinical use, and no interaction with nanoparticles has been confirmed. Here we review the details of these two processes and their effects on the tumor microenvironment. We also focus on the limitations and advantages of each system at both laboratory and commercial scales. The current article discusses how NC and enhanced access and retention affect passive targeting.
Keywords
NP (nanoparticles), EPR (Enhanced permeation and retention), TME (tumor microenvironment), MDR (multiple drug resistance), HCC (hepatic cell carcinoma), SDDS (Smart Drug Delivery Systems)
Introduction
Latest statistics show that liver cancer has become the fifth most common cancer in humans. Asia and Africa have the highest rates of cancer patients, while Europe has the lowest (jemal et al., 2011). Liver disease: Chronic liver disease is a group of diseases that affect the liver in many ways and cause the following diseases: Hepatitis, Alcoholic hepatitis, and Fatty alcohol hepatitis. It has a greater impact worldwide because it causes symptomatic and asymptomatic diseases as well as cancer and death. Ye Y. Integrin, 2011
Current Treatment Challenges
It is well known that treatment is all about early detection, but we have significant limitations in its diagnosis due to consistent and asymptomatic presentation. Ye Y. Integrin, 2011
The concept of cancer treatment is now changing a lot from traditional treatments to specific cancer treatments that can be effective in treatment and reduce the effects of toxic molecules. This change is due to some limitations of traditional medicine.
The Importance of Nanocarrier-Based Therapeutics and Drug Delivery Systems in Solving These Problems
Drug delivery for non-distributed and unregulated drug release. Local delivery of drugs in SDDS can be an alternative treatment option. There are targeting mechanisms, stimulation technology, and smart nanocarriers.
Regarding the biodistribution of chemotherapeutic agents, many studies have shown that the use of nanoparticles can increase drug accumulation in the tumor compared to free drugs due to better photochemical reaction (EPR). This will reduce the side effects of chemotherapy and increase antitumor effects. When targeting a specific tumor, many factors should be taken into consideration, such as selecting the most effective immune targets and creating suitable cells to deliver the drug to the target site without loss during loading Unezaki, K. Maruyama, J. Hosoda, 1996. Therapeutic agents using various nanocarrier systems can act on or target cancer cells. Maeda, T. Sawa, T. Konno, 2001
Purpose and Scope of the Review
Conventional chemotherapy has many side effects including multidrug resistance (MDR), serious side effects, and inappropriate distribution of the drug to multiple sites. Based on the above facts, it has become important to develop new strategies and nanocarriers to ensure that drugs act on specific tumor cells accurately and in the right window.
Overview of Different Types of Nanocarriers (Liposomes, Nanoparticles, Micelles etc.) Used in Cancer Treatment
Multi-targeted, multi-level, and synergistic use of natural components is superior to conventional chemotherapy in the treatment of hepatocellular carcinoma. Literature review shows that natural bioactive components are effective in inhibiting HCC through molecular targeting, increased bioavailability, passive and active targeting, and stimuli-responsive drug delivery exercise. This study provides new therapeutic strategies focusing on the mechanism of action of various natural products and how they affect different molecular pathways in the treatment of HCC. It has also been shown that natural bioactive nanocarriers have beneficial effects in the treatment of HCC. [4]
The increasing use of cancer drugs offers many opportunities for the development of new anticancer drugs. A theoretical perspective is provided to discuss the clinical and economic aspects of current cancer treatment based on EPR results. These are similar to diseases in the body where instead of destroying benign cells like the immune system, malignant cells create an environment that promotes tumor growth. The tumor
microenvironment (TME) is formed by the interaction of malignant and non-malignant cells. It is known that TME plays an important role in all stages of cancer, especially metastasis, immune response, and local resistance. plays an important role. Abnormal cells that have been shown to be associated with TME in human studies generally include lymphatic vessels, tumor vasculature, immune cells, fibroblasts, pericytes, and adipocytes.
Vessels have an irregular shape, irregular shapes, distinct lines, and blind spots. When the tumor reaches the limit of spread, new blood vessels are formed to supply oxygen, remove waste, and take in nutrients. Angiogenesis is the term used to describe the process of forming new blood vessels (the growth of the vascular structure). Tissues without vascular support show the formation of blood vessels, which are pores in the endothelium.
The width can be from 100 nm to 2 ?m, depending on the type and tissue. In addition, lymphatic insufficiency in the tumor vasculature prevents the drainage of intratumoral components, causing them to accumulate in the tissues. The EPR effect is the term used to describe the phenomenon in cancer tissue. The EPR phenomenon can be used as anti-inflammatory and anti-inflammatory drugs. Poly(styrene-maleic acid) NeoCarzinoStatin (SMANCS) is the first anticancer molecule; Through the leaky vascular system, nanoparticles with a smaller diameter than the pores of the tumor enter and remain for a long time.
Bradykinin, peroxynitrite, matrix metalloproteinases, nitric oxide, heme oxygenase, carbon monoxide, tumor necrosis factor-alpha, vascular endothelial growth factor, and others are several vascular mediators and elements involved in the EPR effect. For these reasons, tumors can show the EPR effect.
Introduction to Theranostics and its Applications in Liver Cancer
Cancer diagnosis, blood, and cancer treatment are also performed together with cancer treatment to ensure early diagnosis, accurate molecular diagnosis, timely and appropriate treatment, and emergency intervention. This chapter reviews the state of the art in cancer treatment, including the use of molecular techniques to assess in vivo cancer markers, cancer imaging, and the design and characterization of nanoparticle platforms for therapeutic delivery. Examples of theranostic platforms inspired by light, magnetism, and sound are also included. The challenges in the use of cancer drugs in clinical settings are also discussed. Hepatocellular carcinoma accounts for 75%-80% of all liver diseases and is a leading cause of cancer worldwide, especially in patients with cirrhosis. Since normal tissue is supplied by the portal vein, intravascular treatment such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) is considered a palliative method when the liver tumor is vascularized by high blood vessels. With the developments in radiochemistry and nuclear technology, TARE has become an effective treatment, especially for cancer patients who do not respond to previous treatments or need cancer treatment. Some radionuclides have the necessary physicochemical properties to function as radioembolic agents in the real world. E. Gullotti, Y. Yeo, 2009.
Among them, 90Y is the only one that emits beta rays and is suitable for PET and Bremsstrahlung single photon emission computed tomography (on the other hand, some such as BS SPECT, 131I, 153Sm, 166Ho, 177Lu, 186Re, and 188Re emit both beta and gamma rays, making embolization beads useful for SPECT imaging and treatment. Paired imaging helps guide the liver cancer treatment process during TARE by providing additional diagnostic information. With this in mind, we review theranostic radionuclides that have been or could be used in TARE for cancer, focusing on the most appropriate treatment for clinical use, such as monitoring the content of embolization beads, evaluating the radiation dose, estimating the treatment, and fixing the tare accordingly. E. Gullotti, Y. Yeo, 2009
The five-year survival rate of primary liver cancer is only 10%, making it the second deadliest malignancy worldwide. When diagnosed, most patients are already in the middle and advanced stages, meaning they do not have time for treatment. Radiation or chemotherapy has no effect on the cancer. The current drugs used for liver cancer have disadvantages such as insufficient drug use, short survival time, and drug resistance. Therefore, new diagnostic and therapeutic methods need to be developed to better diagnose, treat, and ultimately predict cancer. Nanobiotechnology provides a new way for accurate diagnosis and treatment in the treatment of liver cancer. Y. Malam, M. Loizidou, A.M. Seifalian, 2009.
Description of Theranostic Platforms Integrating Diagnostic and Therapeutic Components for Personalized Medicine in Liver Cancer
The cells that cause hepatocellular carcinoma (HCC) express the tumor-associated antigen Glypican-3 (GPC3). In this preclinical study, the GPC3-targeting antibody aGPC3 conjugated to zirconium-89 (89Zr) and yttrium-90 (90Y) was utilized to identify, treat, and assess the effectiveness of the theranostic system in mouse models of HCC. creation of orthotopic xenograft mouse models for HCC. Using a small animal PET/CT imaging system (Immuno-PET), animals were injected with 89Zr-labeled aGPC3, and 30 days later, they underwent radioimmunotherapy (RIT) with 90Y-labeled aGPC3. Alpha-fetoprotein (AFP) serum level, a marker of tumor burden, was measured. Immuno-PET measures SUVmax and gross tumor volume (GTV) using fixed density and classification methods. [10]Prior to RIT, immunoPET GTV measurement can be used to quantify the burden and has a strong correlation (R2 = 0.90) with serum AFP.
Mice treated with 90Y-aGPC3 had significantly lower serum AFP levels thirty days after RIT than either untreated (p = 0.01) or non-radiolabeled aGPC3 (p = 0.02). ImmunoPET GTV measurements and post-RIT tumors showed a significant correlation (R2 = 0.87). The GTV of mice given 90Y-aGPC3 treatment was less than that of animals given unradiolabeled aGPC3 treatment or those given no treatment; however, this difference was only statistically significant. A theranostic platform utilizing GPC3 efficiently targets 89Zr and 90Y therapeutically in an HCC xenograft model that expresses GPC3 and assesses the response following radioimmunotherapy. [10]
Describe the challenges (such as anti-inflammatory drugs, resistance issues, and immunogenicity) encountered in developing effective nanomedicines for liver cancer. The off-target toxicity of many drugs can be reduced for the treatment of the disease. However, nano vectors sometimes cause nanotoxicity due to their ability to attack or to cause additional attacks that may weaken the body and have adverse effects. The small size and excellent stability of nanoparticles allow them to reach places that larger particles cannot reach.
Nanoparticles can easily enter cellular organelles such as mitochondria, endoplasmic reticulum, lysosomes, and nuclei through pores in biological tissues. There, they can change the three-dimensional structure of biomolecules by allowing chemical compounds to interact with them. In this way, nanoparticles can cause the body's important enzymes and some hormones to become ineffective. In addition to damage to the heart, lungs, and other tissues and organs, nanoparticles can also cause oxidative stress, inflammatory responses, DNA damage, apoptosis, cell cycle abnormalities, and abnormal gene expression. people listen. Nanostructures with electrical, optical, and magnetic properties can be destroyed, which is obvious and harmless. Nanostructures can enter many bodies and accumulate over time before being removed. They can go to many parts of the body as nanoparticles or can be divided into many parts. Heldin, K. Rubin, K. Pietras, 2004
Unfortunately, because many nanotherapeutics are not disease-specific, damage to healthy tissue cannot be prevented. Although these intelligent drug delivery systems have enormous potential, they also present significant obstacles to the advancement of nanomedicine in the future. They have the potential to eliminate aberrant tissue while leaving healthy tissue intact. Products containing liposome-based nanoformulations include antibiotics, antivirals, and other medications. Sales of doxorubicin (Doxil), the first liposomal nanotherapeutic drug approved by the FDA in 1995, have been rising steadily, reaching an annual sales level of about $600 million. (Doxil sales have decreased as a result of the emergence of generic doxorubicin.) Nonetheless, liposome delivery poses minimal challenges to the reticuloendothelial system (RES) and the mononuclear phagocytic system. Polyethylene glycol, or PEG, has been applied to liposomes in order to decrease liposome clearance; this process, called “PEGylation,” can increase the time a drug remains in the blood.
Discuss Future Directions and Events in this Field
The production and use of materials and devices with dimensions on the nanoscale is called nanotechnology. Examples of nanoparticles, typically 1 to 20 nm in diameter, are quantum dot semiconductor nanocrystals, metal oxide nanoparticles, and colloidal gold. Significant research has been done on these nanotechnologies over the past few years, giving rise to a new field called "nanomedicine."
The last ten years have seen a massive amount of study into these nanotechnologies, giving rise to a new field called "nanomedicine," which is the application of nanotechnology to human health care for the purposes of illness prediction, treatment, monitoring, diagnosis, and prevention. Some of the challenges associated with the application of nanomedicines in human medicine have recently been addressed. Following the FDA's approval of Doxil in the mid-1990s, a number of nanoconstructs are now undergoing
clinical studies and are available for purchase. Nevertheless, there are several barriers to the use of nanoparticles in human applications. A thorough grasp of the chemical and physical characteristics of particles as well as their pharmacokinetic behavior—including their biodistribution, toxicity, and biocompatibility—is required before translating these characteristics into therapeutic usage. We give a general overview of nanomedicines in this review and go over the preclinical and clinical studies that have been conducted to assess them.
Mechanism of Action of Nanocarrier-Based Therapeutics
Theranostic provides shared therapeutic and diagnostic services for cancer diagnosis and treatment to carriers. As seen in Figure 2, this strategy targets the illness at the cellular and molecular level using theranostic nanocarriers. Antibiotics, proteins, peptides, genes, and genetic material are among the therapeutic agents contained in nanocarriers. In the meantime, heavy elements like gadolinium, fluorescent dyes, quantum dots, radionuclides, superparamagnetic metal oxides, and iodine are among the most often used diagnostic medications for nuclear imaging, computed tomography (CT), optical imaging, and magnetic resonance imaging (MRI) (If, 201). For instance, Zhang et al. (2016) employed gadolinium as a contrast agent and lipid micelles as nanocarriers to perform MRI/photoacoustic imaging (PAI) on tumors from HepG2 mice. The selection of tumor cell clones with acidic organelles may be favorably influenced by receiving the actual treatment, which may reduce drug retention and function. If these organelles are part of the secretory system, the drug may exit the cell via exocytosis.
Passive targeting
According to Haley and Frenkel (2008), passive targeting is the process by which nanocarriers are carried through the tumor capillaries' leaky fenestrations and enter the tumor interstitium and cells by passive diffusion or convection. Convection is the term used to describe the movement of molecules in fluids. It is mainly used to describe the movement of large molecules through large pores when there is no net filtration rate. Low molecular weight substances, on the other hand, such as oxygen, mostly rely on diffusion, which is the movement of molecules across cell membranes that are fueled by concentration gradients and occur without the need for cellular energy. Diffusion is the main method of drug transport, though, because interstitial hypertension prevents convection within the tumor interstitium.
Passive targeting of nanocarriers involves two main concepts. First, nanocarriers selectively reach the tumor by using the permeable vascular system surrounding the tumor. Second, they use the enhanced permeability and retention (EPR) effect to be retained in the tumor. Unlike free-diffusing drugs, they quickly concentrate in blood vessels due to their small size, while drugs containing nanocarriers cannot return to blood vessels due to their larger size. This causes them to gradually accumulate in the tumor. This selective binding promotes the accumulation of nanocarriers and drugs in tumors via the EPR effect (Haley and Frenkel, 2008). Many nanocarriers exploit this phenomenon. They are particularly effective against rapidly growing tumors, resulting in an increase in the local area of drug-loaded nanocarriers in tumors compared to normal tissue, reaching 10–50 times the local area of drug-loaded nanocarriers in tumors, respectively, within 1–2 days (Iyer et al. ., 2006). However, the effectiveness of the EPR effect depends on the ability of nanocarriers to evade the immune system and maintain long-term circulation.
1. Size: The largest size of nanocarriers is in the range of 10 to 100 nm. This size facilitates effective penetration into tumor cells while preventing kidney damage (size greater than 10 nm) and liver-specific capture (size greater than 100 nm).
2. Charge: Nanocarriers must have a neutral or anionic charge to escape renal elimination. There are some limitations to this goal:
Dependence on Tumor Vascularization and Angiogenesis
The effectiveness of passive targeting varies depending on tumor vascularization and angiogenesis, resulting in different nanocarrier concentrations in different tumor types and anatomical regions (Bae, 2009).
High Interstitial Fluid Pressure in the Tumor
Medication distribution and intratumoral penetration are enhanced by elevated interstitial fluid pressure within the tumor. The relationship between this pressure and the EPR effect is affected by both low fluidity and larger, longer-length nanocarriers (100 nm) which are more likely to deposit in tumors while smaller molecules are more likely to diffuse away (Pirollo and Chang, 2008).
Figure 1
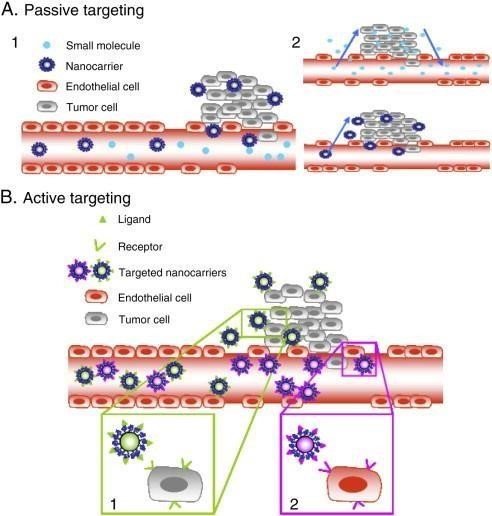
Active Targeting
In active targeting, certain receptors expressed at the target site are bound by ligands attached to the surface of nanocarriers. These ligands are chosen so that they only attach to receptors that are overexpressed in tumor cells or the tumor vasculature, and so do not attach to normal cells. Targeted receptors should also express themselves uniformly in all targeted cells. Monoclonal antibodies (mAbs) and antibody fragments, as well as non-antibody ligands that can be either peptidyl or non-peptidyl in nature, can be utilized as ligands for targeting. The "binding-site barrier" is a notion that affects the binding affinity of these ligands, which in turn affects tumor penetration. Because of the bloodstream's dynamic flow environment, high-affinity binding is frequently preferred in targets where cells are easily accessible, such as tumor vasculature (Adams et al., 2001; Gosk et al., 2008).
Biological Barriers and Strategies for Overcoming Them
Biological barriers present serious obstacles to the effective delivery of nanotherapeutics to diseased sites, making it more difficult to treat a variety of illnesses, from inflammation to cancer. Many strategies fail to overcome these obstacles, despite significant research efforts to improve nanoparticle design with multiple functionalities and moieties. Problems like non-specific distribution and inadequate accumulation of treatments continue to be major roadblocks for pharmaceutical companies. In order to overcome these obstacles and achieve site-specific delivery of therapeutics, a fundamental rethinking of conventional nanoparticle design is necessary.
Without comprehensive consideration of the biological barriers encountered upon intravenous administration, the realization of site-specific drug delivery will remain elusive. It is crucial to address the majority, if not all, of these barriers in a systematic manner during nanoparticle design. By tackling each barrier successively, innovative design features can be strategically integrated, ushering in a new era of nanotherapeutics and revolutionizing nanoparticle-based drug delivery. It's important to emphasize that failure to adequately address the majority if not all, biological barriers during nanoparticle design will impede the clinical potential of nanoparticle-based drug delivery.
The Mononuclear Phagocyte System's Opsonization/Sequestration Mechanism
The primary barrier to the delivery of nanotherapeutics is reaching therapeutic drug levels at disease sites, primarily due to the nonspecific uptake of nanoparticles in healthy organs. The Mononuclear Phagocyte System (MPS), which is composed of phagocytic cells—specifically, resident macrophages in the liver, lymph nodes, and spleen—quickly sequesters nanoparticles following injection (Patel & Moghimi, 1998). Opsonization of nanoparticles is the initial stage of this sequestration process, whereby circulating nanoparticles adsorb plasma proteins like immunoglobulins, complement components, serum albumin, and apolipoproteins (Tenzer, 2013).
Hemorheology and Blood Vessel Fluid Dynamics
The size and geometry of nanoparticles have a significant impact on their fluid dynamics in blood vessels. Conventional nanoparticle delivery systems, such as liposomes and polymer nanoparticles, usually have a spherical shape, with diameters between 10 and 100 nm, and are designed mainly for intravenous administration. In an effort to improve the way that nanoparticles interact with vessel walls and ease their extravasation to disease sites, a number of strategies have been developed to improve the margination of nanoparticles. New directions in nanoparticle geometry have been investigated recently, deviating from the traditional spherical forms.
Extravasation of Nanoparticles and Intratumoral Pressure
The potential of nanoparticles as a drug delivery system to concentrate precisely at sites of inflammation, infection, and injury is a major factor driving the excitement surrounding this technology. The fundamental study on the Enhanced Permeability and Retention (EPR) effect by Maeda and colleagues shows that endothelial dysfunction and blood vessel fenestrations are the main causes of this passive targeting ability (Matsumura & Maeda, 1986; Maeda & Nakamura, 2013). This phenomenon is particularly evident in cancer, where the aggressive angiogenic nature of tumors leads to a disorganized and chaotic vasculature. This feature is not specific to cancer, but it has prompted the creation of tactics that aim to disrupt angiogenic vessels in order to enhance the concentration of nanoparticles at particular locations.
Passage of the Cellular Membrane and the Ensuing Endosomal Compartmentalization
The excitement surrounding this technology is largely due to the potential of nanoparticles as a drug delivery system that can concentrate precisely at sites of inflammation, infection, and injury. Maeda et al. (2009) conducted a fundamental study on the Enhanced Permeability and Retention (EPR) effect, which indicates that the primary causes of this passive targeting ability are endothelial dysfunction and blood vessel fenestrations. This phenomenon is especially noticeable in cancer, as the vasculature becomes disorganized and chaotic due to the aggressive angiogenic nature of tumors. This characteristic is not unique to cancer, but it has led to the development of strategies designed to interfere with angiogenic vessels in order to increase the concentration of nanoparticles at specific sites.
Drug Efflux Pumps are a Source of Multidrug Resistance
One of the biggest obstacles to treating many disease processes, such as cancer, inflammation, and infection, is drug resistance. Drugs are ejected from cells by multidrug resistance (MDR), which can be intrinsic or acquired through repeated exposure. This lowers intracellular concentrations of the drugs and decreases the effectiveness of therapy. As seen with anthracyclines, taxanes, and vinca alkaloids, cancerous cells develop resistance to chemotherapeutics regardless of the drug's structure or mode of action (Szakács et al., 2006). Because healthy cells are exposed to the drug being expelled, this causes increased local toxicity and calls for higher treatment doses, which frequently cause severe patient morbidity. As a result, nonresponsive recurrence and the consequent failure of particular chemotherapy regimens are possible outcomes.
Imaging Techniques for Monitoring Nanodrug Delivery and Therapeutic Response
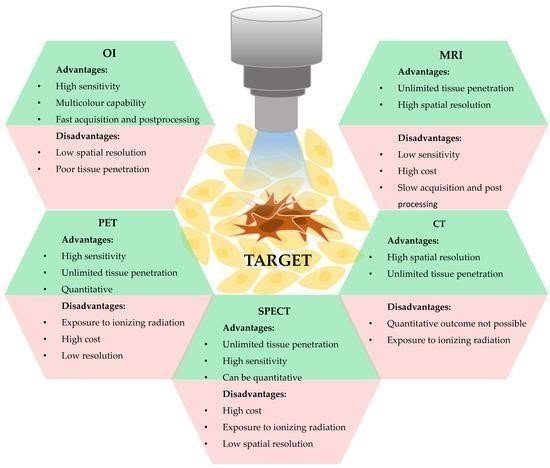
The relative benefits and drawbacks of various imaging modalities affect which ones are appropriate for use in nanotheranostic applications. Since it can contrast soft tissue without requiring tissue penetration and has a high spatial resolution, magnetic resonance imaging (MRI) is a frequently used diagnostic tool.
Conversely, MRI is less sensitive, more costly, and takes longer. Magnetic resonance imaging (MRI) offers a higher spatial resolution and is less expensive than radionuclide imaging (PET/SPECT), despite having unlimited tissue penetration and high sensitivity. CT provides high spatial resolution and deep tissue penetration, despite the risks of ionizing radiation exposure. Fluorescence optical imaging (OI) allows for compound optimization, target confirmation, and high-throughput screening due to its high sensitivity and multi-color imaging capabilities. However, OI's spatial resolution is inadequate.
It is imperative to take these factors into account when choosing the best imaging modality to achieve desired outcomes.
Figure 2
(Arms et el 2018).
Optical imaging (OI) provides a noninvasive method for imaging organs and tissues using light and is easy to use compared to other procedures. Its cost-effectiveness also increases its appeal. However, unless fluorescent paper is used, the absorption and scattering properties of body tissue that OI shows are not sufficient to provide detailed information about the study.
Auto-fluorescence and deep penetration are difficult for us, limiting their clinical applications mainly to research studies. Recent developments have addressed some of these limitations, increasing the clinical benefits of fluorescence imaging. Endoscopic examination of the cervix and lungs, where neoplastic changes are associated with loss of fluorescence. Despite its deep penetration, OI has proven useful in detecting diseases in many organs, including the breast, cervix, trachea, intestine, skin, prostate, brain, and blood glands. The group is not currently publishing for surface methods, but near-infrared probes enable deep detection, making them promising for in vivo applications.
Techniques such as 2D fluorescence reflectance imaging are suitable for shallow tissues, while fluorescence molecular tomography (FMT) allows 3D imaging up to 12 cm deep. Super-resolution microscopy techniques such as stimulated emission depletion (STED) have also emerged as promising in vivo imaging tools due to their 3D slicing capabilities. This is particularly useful for guidance in tumor resection surgery. Cancer development can affect patient survival, especially in cases such as glioma, where tissue removed during surgery is important. Penetrating fluorescence imaging provides another method for depth recording. This approach allows advanced microscopy techniques, including high-resolution microscopy, to be used to analyze particles in detail. Nanotherapy platform.
Nanomaterials as Probes for In-Vivo Optical Imaging?
Cancer treatment presents complex challenges and often requires multiple approaches to achieve good results. In some cases, surgery is better, but for patients with contraindications to surgery, there are a number of interventions available, including chemotherapy and radiation therapy, including photothermal therapy (PTT) and photodynamic therapy (PDT). Chemotherapy and radiation therapy are cancer treatments designed to interfere with cell division and DNA replication/transcription, respectively. However, they have mild side effects, are well-managed, and are often used in combination with other treatments. Gene therapy is an emerging field that directly targets tumors and can provide long-term results, but it also requires careful consideration of genetic modification. PDT uses light-activated photosensitizers to selectively activate tumor cells with minimal toxicity. For example, PTT directly kills cancer cells by converting light energy into heat and often uses nanoparticle-based photothermal agents to precisely target and reduce nasal inflammation.
PTT or PDT and chemotherapy have been shown to be synergistic in the treatment of breast cancer.
Nanotechnology plays a key role in this regard, facilitating drug delivery, gene therapy, and imaging.
Nanomaterials provide opportunities for drug delivery by increasing efficacy while reducing side effects. Advances in nanotechnology promise to improve clinical outcomes through drug delivery and integration, underscoring the importance of collaboration in healthcare.
Imaging-Guided Surgery and Drug Delivery?
Because fluorescence imaging can selectively label tumor cells, it can be used to guide surgeons during tumor extraction surgeries. There may be uses for methods like resecting cancerous nodes that involve injecting mice with quantum dots (QDs) to track lymphatic drainage.
Additionally, by differentiating between healthy and unhealthy tissue, activated cell-penetrating peptides have been developed to guide surgical resection, especially in cases of glioblastoma. Furthermore, sections of breast tumors have been imaged using QDs decorated with multiple biomarkers to aid in tissue analysis. Furthermore, nanomaterials enable multimodal imaging, which combines fluorescence imaging with other techniques like MRI, photoacoustic imaging, Raman spectroscopy imaging, PET, or SPECT. The biodistribution and tumor accumulation studies of polymeric nanocarriers demonstrate that combinations such as CT combined with fluorescence molecular tomography (FMT) provide improved spatial reference. With the help of this multimodal imaging approach, therapeutic doses can be optimized and drug over- or under-dosing can be prevented. It also provides comprehensive information on tissue progression, drug delivery kinetics, and treatment efficacy.
Figure 3

Treatment Monitoring
Diagnosis and Imaging of Breast Cancer Profiling of Biomarke
The use of quantum dots (QDs) in molecular analysis has advantages over traditional methods, particularly in immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). Quantum dots have unique optical properties, including excellent image quality, allowing them to fluoresce for long periods of time without rapid decay (photobleaching). This ability enables accurate measurement of protein behavior by spectrometry since the emission spectrum of each QD color depends on its size and provides a known peak wavelength for detection. Conjugation of specific antibodies allows simultaneous detection of multiple tumor cells on a single tumor sample. This multiplexing process increases sensitivity and specificity in detecting low-level targets in cancer cells, thereby improving diagnostic accuracy. Studies have demonstrated the effectiveness of QD-based assays in detecting the abundance of estrogen receptors, progesterone receptors, and ERBB2 in breast cancer cells and tumors, in relation to chemical IHC and Western blotting. Additionally, QDs are promising in investigating gene expression or RNA distribution in FISH applications. Compared with organic fluorescent molecules, QD-labeled oligonucleotide probes exhibit bright and stable fluorescence, which facilitates the detection and measurement of gene expression. Quantum dot-based FISH methods are more effective than traditional methods, especially in determining low-level gene expression. They provide rich spectral information under ambient conditions. Silica shell-coated surface-enhanced Raman scattering (SERS) probes provide enhanced illumination and rich spectral information, expanding the possibilities for labeling various biomarkers in tissue samples. However, there are still some challenges before QD-based technology can be widely used in surgical practice. Optimizing bioconjugation methods and developing cost-effective spectroscopic microscopy for precise measurement are important steps to overcome these problems. However, quantum dot conjugates and Raman probes are capable of simultaneous quantification of multiple proteins in single tumors or small tumor samples, thus guiding treatment decision-making based on molecular profile information.
Treatment of Liver Cancer
Cancer treatment presents complex challenges and often requires multiple approaches to achieve good results. In some cases, surgery is better, but for patients with contraindications to surgery, there are a variety of interventions available, including radiation therapy and chemotherapy, in addition to photothermal therapy (PTT) and photodynamic therapy (PDT).
Cancer treatments such as chemotherapy and radiation therapy target the processes of cell division and DNA replication/transcription, respectively.
However, their side effects are not good and can be managed carefully and are often combined with other modifications. Gene therapy is an emerging field that directly targets the tumor, providing long-term results but also requires careful consideration of genetic modification. PDT uses photosensitizers that are activated by light to selectively activate tumor cells with minimal toxicity. In contrast, PTT directly kills tumor cells by converting light energy into heat, often using nanoparticle-based photothermal agents to precisely target and reduce the healthy nose. PTT or PDT and chemotherapy have been shown to have synergistic potential in cancer treatment.
Nanotechnology plays a key role in this regard, facilitating drug delivery, gene therapy, and imaging.
Nanomaterials provide an opportunity for drug delivery by increasing efficacy while reducing side effects. Disruption. Advances in nanotechnology promise to improve medical outcomes through drug delivery and integration, and underscore the importance of collaboration in healthcare.
Applications
Applications in Regenerative Medicine and Tissue Engineering
Tissue engineering and regenerative medicine hold great potential for biomedical applications that combine biological processes with structural engineering to facilitate the regeneration of tissues, including organ-bearing tissues. An essential part of developing biocompatible and biodegradable materials for tissue engineering and regenerative medicine is the use of biopolymers, particularly stimuli-responsive biopolymers. Alginates are one type of biopolymer that is frequently used to make scaffolds and structures that respond to stimuli.
These materials can be designed to exhibit specific physical properties and materials by creating porous materials and films with desired patterns. These scaffolds provide critical support for cellular functions such as growth, differentiation, and migration required for tissue formation, therapy, and other diseases. Stimuli-responsive nanocarriers such as polymeric nanocarriers have been developed for targeted drug delivery. These nanocarriers respond to environmental signals such as pH and redox conditions, allowing the drug to be released in any cellular environment. The role of tissue regeneration demonstrates its potential in regenerative medicine. Nanoparticles aggregate in the tumor microenvironment and release the drug according to specific conditions, indicating efficacy against cancer cells while reducing toxicity. Stellate cell proliferation can be inhibited and neuronal culture differentiation regulated by over-optimization. By modifying cellular interactions and biochemical processes, these modified hydrogels have the potential to treat diseases of the central nervous system. The approach has a lot of potential for offering efficient treatment programs for different illnesses and ailments.
In-Vivo Applications
Numerous variables, including the kind of nanocarrier, ultrasound parameters, drug type, tumor characteristics, and treatment, are involved in in vivo application and impact the course of treatment. Table 3 gives a summary of foreign US applications using different US nanocarriers along with the in vivo clinical outcomes, expressed as volume inhibition rate (VIR) or percent tumor reduction (TVR) relative to non-US treatments. Although substantial tumor inhibition (>50%) can occasionally be obtained without ultrasonography, ultrasonography-assisted treatment is always superior or equivalent. Tumor regression will, however, take longer to ideally reach 100%.
This outcome was attained in three different ways by the studies:
1** Khairolomoom and associates [145]: ** By combining ultrasound-mediated hyperthermia with hot liposomes (TSL), they were able to completely eradicate NDL breast cancer in mice. These liposomes include pH-sensitive copper doxorubicin (CuDOX) complexes, which release free DOX when they dissociate in a low-pH environment. Eight months after treatment, all mice given CuDOX-LTSL plus US were still alive and tumor-free.
2**Liang & Associates [146]: ** Using liposomal ceramic bodies in conjunction with liver-focused ultrasound (HIFU), they were able to achieve 96% TVR of human breast cancer xenografts in mice. Long-term blood circulation and healing can be facilitated by the temperature-responsive nanohybrid ceramic body created by integrating lipid components of LTSL into the ceramic body.
3 **Snippstadt and associates [147]: ** They utilized focused ultrasound (FUS) in conjunction with nanoparticle-stabilized microbubbles (MB) to achieve total and stable regression of human breast cancer xenografts in mice. It has been demonstrated that an average sound pressure of MI 0.5 can treat cancer without causing tissue damage, in contrast to the use of ultrasonography. Large tumor volume inhibition rates (>50%) were observed with metallipid nanoparticles, mesoporous nanoparticles, silica nanoparticles loaded with drugs and sonosensitizers, and chemotherapeutic drug-loaded membrane fusion liposomes.
Conclusion
The ability of nanocarriers to transform the treatment of liver cancer is clear and provides solutions to many of the challenges faced by conventional therapy. Nanocarriers have the advantages of high drug packaging, good stability, controlled release and delivery, and can increase the efficacy of anti-inflammatory drugs and reduce side effects. These problems can be realized to the benefit of nanocarriers in the treatment of advanced cancer. The biggest challenge is the overabundance of nanocarriers in the liver, which may hinder their ability to target cancer cells. Strategies to increase the specificity of nanocarriers to cancer cells while minimizing off-target effects are important.
Basic nanocarriers This will involve the development of new targets or ligands to facilitate the transfer of nanocarriers to specific sites of the tumor. Diverse physicochemical properties in nanocarriers. This requires continued research to identify and optimize nanomedicines with improved therapeutic and imaging sensitivity. Designing nanomaterials that enable precise control of drug release kinetics could improve clinical outcomes and reduce toxicity. The use of nanotherapeutic systems is crucial. To overcome these obstacles and fully utilize nanomedicine in the treatment of cancer, more research and innovation in this field will be required.
References
-
Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. 2001) High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules.
- Bae, Y. H. (2009). Drug targeting and tumor heterogeneity. Journal of Controlled Release, 133(1), 2–3. https://doi.org/10.1016/j.jconrel.2008.09.074
- Gosk, S., Moos, T., Gottstein, C., & Bendas, G. (2008). VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochimica Et Biophysica Acta (BBA) - Biomembranes, 1778(4), 854–863. https://doi.org/10.1016/j.bbamem.2007.12.021
- Gullotti, E., & Yeo, Y. (2009). Extracellularly Activated nanocarriers: a new paradigm of tumor targeted drug delivery. Molecular Pharmaceutics, 6(4), 1041–1051. https://doi.org/10.1021/mp900090z
- Haley, B., & Frenkel, E. (2008). Nanoparticles for drug delivery in cancer treatment. Urologic Oncology Seminars and Original Investigations, 26(1), 57–64. https://doi.org/10.1016/j.urolonc.2007.03.015
- Heldin, C., Rubin, K., Pietras, K., & Östman, A. (2004). High interstitial fluid pressure — an obstacle in cancer therapy. Nature Reviews. Cancer, 4(10), 806–813. https://doi.org/10.1038/nrc1456
- Iyer, A. K., Khaled, G., Fang, J., & Maeda, H. (2006). Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today, 11(17–18), 812–818. https://doi.org/10.1016/j.drudis.2006.07.005
- Maeda, H., Bharate, G. Y., & Daruwalla, J. (2009). Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics, 71(3), 409–419. https://doi.org/10.1016/j.ejpb.2008.11.010
- Maeda, H., Sawa, T., & Konno, T. (2001). Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. Journal of Controlled Release, 74(1–3), 47–61. https://doi.org/10.1016/s0168-3659(01)00309-1
- Malam, Y., Loizidou, M., & Seifalian, A. M. (2009). Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends in Pharmacological Sciences, 30(11), 592–599. https://doi.org/10.1016/j.tips.2009.08.004
-
Matsumura, Y., & Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research, 46(12), 6387–6392.
- Moghimi, S., & Patel, H. (1998). Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system – The concept of tissue specificity. Advanced Drug Delivery Reviews, 32(1–2), 45–60. https://doi.org/10.1016/s0169-409x(97)00131-2
- Pirollo, K. F., & Chang, E. H. (2008). Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends in Biotechnology, 26(10), 552–558. https://doi.org/10.1016/j.tibtech.2008.06.007
- Szakács, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C., & Gottesman, M. M. (2006). Targeting multidrug resistance in cancer. Nature Reviews Drug Discovery, 5(3), 219–234. https://doi.org/10.1038/nrd1984
- Tenzer, S., Docter, D., Kuharev, J., Musyanovych, A., Fetz, V., Hecht, R., Schlenk, F., Fischer, D., Kiouptsi, K., Reinhardt, C., Landfester, K., Schild, H., Maskos, M., Knauer, S. K., & Stauber, R. H. (2013). Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature Nanotechnology, 8(10), 772–781. https://doi.org/10.1038/nnano.2013.181
- Unezaki, S., Maruyama, K., Hosoda, J., Nagae, I., Koyanagi, Y., Nakata, M., Ishida, O., Iwatsuru, M., & Tsuchiya, S. (1996). Direct measurement of extravasation of polyethyleneglycol-coated liposomes into solid tumor tissue by in vivo fluorescence. International Journal of Pharmaceutics, 144(1), 11–17.
- Zhang, D., Wu, M., Zeng, Y., Liao, N., Cai, Z., Liu, G., Liu, X., & Liu, J. (2016). Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. Journal of Materials Chemistry B, 4(4), 589–599. https://doi.org/10.1039/c5tb01827g
-
Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM. 2001) High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules.
- Bae, Y. H. (2009). Drug targeting and tumor heterogeneity. Journal of Controlled Release, 133(1), 2–3. https://doi.org/10.1016/j.jconrel.2008.09.074
- Gosk, S., Moos, T., Gottstein, C., & Bendas, G. (2008). VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochimica Et Biophysica Acta (BBA) - Biomembranes, 1778(4), 854–863. https://doi.org/10.1016/j.bbamem.2007.12.021
- Gullotti, E., & Yeo, Y. (2009). Extracellularly Activated nanocarriers: a new paradigm of tumor targeted drug delivery. Molecular Pharmaceutics, 6(4), 1041–1051. https://doi.org/10.1021/mp900090z
- Haley, B., & Frenkel, E. (2008). Nanoparticles for drug delivery in cancer treatment. Urologic Oncology Seminars and Original Investigations, 26(1), 57–64. https://doi.org/10.1016/j.urolonc.2007.03.015
- Heldin, C., Rubin, K., Pietras, K., & Östman, A. (2004). High interstitial fluid pressure — an obstacle in cancer therapy. Nature Reviews. Cancer, 4(10), 806–813. https://doi.org/10.1038/nrc1456
- Iyer, A. K., Khaled, G., Fang, J., & Maeda, H. (2006). Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today, 11(17–18), 812–818. https://doi.org/10.1016/j.drudis.2006.07.005
- Maeda, H., Bharate, G. Y., & Daruwalla, J. (2009). Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. European Journal of Pharmaceutics and Biopharmaceutics, 71(3), 409–419. https://doi.org/10.1016/j.ejpb.2008.11.010
- Maeda, H., Sawa, T., & Konno, T. (2001). Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. Journal of Controlled Release, 74(1–3), 47–61. https://doi.org/10.1016/s0168-3659(01)00309-1
- Malam, Y., Loizidou, M., & Seifalian, A. M. (2009). Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends in Pharmacological Sciences, 30(11), 592–599. https://doi.org/10.1016/j.tips.2009.08.004
-
Matsumura, Y., & Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research, 46(12), 6387–6392.
- Moghimi, S., & Patel, H. (1998). Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system – The concept of tissue specificity. Advanced Drug Delivery Reviews, 32(1–2), 45–60. https://doi.org/10.1016/s0169-409x(97)00131-2
- Pirollo, K. F., & Chang, E. H. (2008). Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends in Biotechnology, 26(10), 552–558. https://doi.org/10.1016/j.tibtech.2008.06.007
- Szakács, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C., & Gottesman, M. M. (2006). Targeting multidrug resistance in cancer. Nature Reviews Drug Discovery, 5(3), 219–234. https://doi.org/10.1038/nrd1984
- Tenzer, S., Docter, D., Kuharev, J., Musyanovych, A., Fetz, V., Hecht, R., Schlenk, F., Fischer, D., Kiouptsi, K., Reinhardt, C., Landfester, K., Schild, H., Maskos, M., Knauer, S. K., & Stauber, R. H. (2013). Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature Nanotechnology, 8(10), 772–781. https://doi.org/10.1038/nnano.2013.181
- Unezaki, S., Maruyama, K., Hosoda, J., Nagae, I., Koyanagi, Y., Nakata, M., Ishida, O., Iwatsuru, M., & Tsuchiya, S. (1996). Direct measurement of extravasation of polyethyleneglycol-coated liposomes into solid tumor tissue by in vivo fluorescence. International Journal of Pharmaceutics, 144(1), 11–17.
- Zhang, D., Wu, M., Zeng, Y., Liao, N., Cai, Z., Liu, G., Liu, X., & Liu, J. (2016). Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. Journal of Materials Chemistry B, 4(4), 589–599. https://doi.org/10.1039/c5tb01827g
Cite this article
-
APA : Zahid, D., Tahir, R., & Ashfaq, W. (2024). Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Global Pharmaceutical Sciences Review, IX(II), 18-29. https://doi.org/10.31703/gpsr.2024(IX-II).03
-
CHICAGO : Zahid, Dua, Rabia Tahir, and Wajiha Ashfaq. 2024. "Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities." Global Pharmaceutical Sciences Review, IX (II): 18-29 doi: 10.31703/gpsr.2024(IX-II).03
-
HARVARD : ZAHID, D., TAHIR, R. & ASHFAQ, W. 2024. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Global Pharmaceutical Sciences Review, IX, 18-29.
-
MHRA : Zahid, Dua, Rabia Tahir, and Wajiha Ashfaq. 2024. "Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities." Global Pharmaceutical Sciences Review, IX: 18-29
-
MLA : Zahid, Dua, Rabia Tahir, and Wajiha Ashfaq. "Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities." Global Pharmaceutical Sciences Review, IX.II (2024): 18-29 Print.
-
OXFORD : Zahid, Dua, Tahir, Rabia, and Ashfaq, Wajiha (2024), "Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities", Global Pharmaceutical Sciences Review, IX (II), 18-29
-
TURABIAN : Zahid, Dua, Rabia Tahir, and Wajiha Ashfaq. "Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities." Global Pharmaceutical Sciences Review IX, no. II (2024): 18-29. https://doi.org/10.31703/gpsr.2024(IX-II).03
