Abstrict
Study and evaluation of the prevalence of measles and its complication in vaccinated and non-vaccinated patients. To evaluate the prevalence of complications of measles among different age groups of children in the pedriatric ward. Clinical description of a case series. Children of different age groups suffering from measles. We have observed 315 measles patients in the pediatric ward of Bahawal Victoria Hospital in Bahawalpur. They were evaluated on the basis of complications. Different complications were observed in children of different age group and gender, and the main complications were encephalitis, conjunctivitis, pneumonia, diarrhoea and ear infections. Children of age less than 1 year to 7 years were observed, and it was observed that conjunctivitis, pneumonia and diarrhoea were among the most common complications, while encephalitis and ear infections were rare. Patients admitted to the pedriatric ward having long term complications have a high risk of death when treated. These patients were due to malnutrition and improper vaccination coverage, and improper vaccine storage. Respiratory distress, i.e., pneumonia, diarrhoea, conjunctivitis, ear infection and encephalitis, were the most common complications. To reduce the severity of these complications, mechanical ventilation, antibiotics treatment, electrolyte balance should be instituted early in patients with measles.
Keywords
Measles, Vaccine, Patients
Introduction
Measles is an acute viral infectious disease. References to measles can be found as early as the 7th century. The disease was described by the Persian physician Rhazes in the 10th century as “more dreaded than smallpox.” [1].
The incubation period of measles and lifelong immunity after recovery from the measles was described by Peter Panum in 1846, who isolated the virus in monkey and human kidney tissue culture in 1954 [2]
In the US in 1963, the first live attenu¬ated vaccine (Edmonston B strain) was licensed for use. Before the availability of the vaccine, measles infection was nearly universal during childhood, and about 90% of infected person were immune by the age of fifteen years. Measles is still fatal and commonly occurred disease in developing countries. WHO estimates 164,000 deaths from measles in 2008 [3-5].
The measles virus is a genus Morbillivirus, paramyxovirus. Its diameter is about 100–200 nm with a core of single-stranded RNA and is found to be closely related to the rinderpest and canine distemper viruses. In pathogenesis, two membrane envelope proteins are essential. They are the F (fusion) protein, which is responsible for the fusion of the virus and the H (hemagglutinin) protein, which is responsible for cell adsorption of the virus [6-8].
There is only one antigenic type of measles virus. Although studies have documented variations in the H glycoprotein, these alterations do not look to be epidemiologically essential (i.e., no change in vaccine efficacy has been observed). Measles virus is rapidly inactivated by light, heat, trypsin, ether and acidic pH. It has a short survival time (less than 2 hours) in the air or on surface and objects [9].
Methodology
We visited Bahawal Victoria hospital Bahawalpur, and we have seen 315 patients with measles during our visit to the pediatric ward in the hospital from 15th April to 30th May 2013. These patients were of different age groups among children. After taking the history of patients, we observed patients suffering from different types of complications of measles such as pneumonia, conjunctivitis, diarrhoea, otitis media, encephalitis.
Pneumonia was the most common complication among them. About 116 children of different age groups were suffering from this complication. Then other very common complication was conjunctivitis, and 86 patients were suffering from conjunctivitis in measles. Diarrhoea was also observed among these children, and about 73 patients were affected by diarrhoea. Other complications among these measles patients were also observed, but their ratio was not significant [10].
History Taking
|
Patient No. |
Age |
Sex |
Present Complaints |
Causes |
Onset Of Disease |
Complications |
|||
|
Pneumonia |
Diarrhea |
conjunctivitis |
Otitis media |
||||||
|
1 |
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
|
|
10 |
|
|
|
|
|
|
|
|
|
Result and Discussion
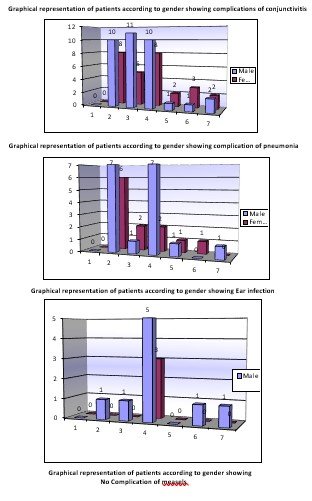
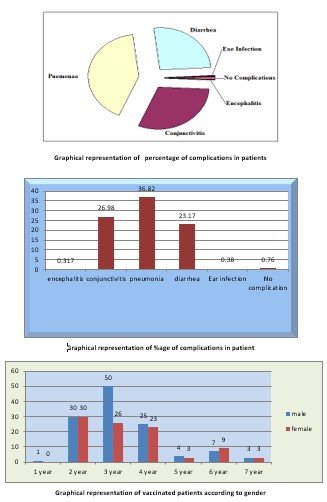
The large body of literature on measles complications has shown consistently that the population is largely effected by complications caused by measles, which may lead to death. The patient groups include the children with prior socioeconomic status, and most were vaccinated with the MMR vaccine of measles. Different complications were observed in children of different age group and gender, and the main complications were encephalitis, conjunctivitis, pneumonia, diarrhoea and ear infections. Children of age less than 1 year to 7 years were observed, and it was observed that conjunctivitis, pneumonia and diarrhoea were among the most common complications, while encephalitis and ear infections were rare. An only a small number of patients were without any complications. Males patients were 175 while the female was 137, which shows that male were more susceptible to complications than females. According to the percentage of 0.31%, patients were suffering from encephalitis. 26.98% of patients were suffering from conjunctivitis, 36.82% patients were suffering from pneumonia, 23.17% of patients were suffering from diarrhoea, 0.38% patients were suffering from ear problems, and only0.76%patients were without any complications. Data also showed that privilege of complications due to measles was more in the children of age 1-4 year, and with increasing age, the number of patients decreases [11].
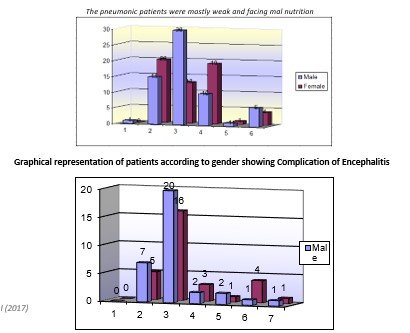
Among the 312 patients, it was also observed that what number of patients were already vaccinated or not with the MMR vaccine of measles. It was observed that 214 patients were vaccinated, while 98 patients were non-vaccinated. Among vaccinated patients, 120 were males while 94 were females, while among non-vaccinated, 55 were male and 43 were females. Measles in vaccinated patients may be due to the failure of vaccines. Most patients were from low socioeconomic status. The pneumonic patients were mostly weak and facing mal nutrition [12].
Different complications were observed in children of different age group and gender, and the main complications were encephalitis, conjunctivitis, pneumonia, diarrhoea and ear infections. Children of age less than 1 year to 7 years were observed, and it was observed that conjunctivitis, pneumonia and diarrhoea were among the most common complications, while encephalitis and ear infections were rare. Only a small number of patients was without any complications. Males patients were 175 while the female was 137, which shows that male were more susceptible to complications than females. According to the percentage of 0.31%, patients were suffering from encephalitis. 26.98% of patients were suffering from conjunctivitis, 36.82% patients were suffering from pneumonia, 23.17% of patients were suffering from diarrhoea, 0.38% patients were suffering from ear problems, and only0.76%patients were without any complications. Data also showed that privilege of complications due to measles was more in the children of age 1-4 year, and with increasing age, the number of patients decreases. Among the 312 patients, it was also observed that what number of patients were already vaccinated or not with MMR vaccine of measles. It was observed that 214 patients were vaccinated, while 98 patients were non-vaccinated. Among vaccinated patients, 120 were males while 94 were females, while among non-vaccinated 55 were male and 43 were females. Measels in vaccinated patients may be due to failure of vaccines [13].
One change in the pattern of measles after the impact of mass vaccination is the shift in cases to adulthood and the appearance of cases in non-vaccinated infants aged <16months, who accounted for a substantial part of the reported cases in 2006-2008, when the infection affected unvaccinated children from a day-care centre, changing the age pattern; cases in this age group rose from 25% in 2001-2005 to 35% in 2006-2008. However, in 2008 there were cases in children aged 4-19 years, an age group in which measles had been practically non-existent [14].
Conclusion
Different complications appear due to measles in children of young age in which conjunctivitis pneumonia, and diarrhoea were among the most common complications while encephalitis and ear infections were among rare complications. Most patients were vaccinated.
Inadequate coverage of MMR vaccination of children below 10 years, probably due to parental illiteracy, the bias in the primary evaluation of the target population, warns the provincial health system for better programming of immunization in the coming years and active and persistent evaluation to detect sporadic cases of diseases among the non-vaccinated population. Considering the low complication rate of mass campaign MR vaccination with high vaccination coverage, it seems that the nationwide organized system to implement mass campaign MR vaccination and follow up its complication was adequate for the achievement of greets goal of eradication of measles and rubella.
|
Age (Years) |
Encephalitis |
Conjunctivitis |
Pneumonia |
Diarrhea |
Ear infection |
No Complication |
||||||||||||
|
M |
F |
Total |
M |
F |
Total |
M |
F |
Total |
M |
F |
Total |
M |
F |
Total |
M |
F |
Total |
|
|
?1 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
15 |
20 |
35 |
7 |
5 |
12 |
3 |
2 |
5 |
10 |
8 |
18 |
7 |
6 |
13 |
1 |
0 |
1 |
|
3 |
30 |
13 |
43 |
20 |
16 |
36 |
8 |
6 |
14 |
11 |
5 |
16 |
1 |
2 |
3 |
1 |
0 |
1 |
|
4 |
10 |
9 |
19 |
2 |
3 |
5 |
4 |
6 |
10 |
10 |
8 |
18 |
7 |
2 |
9 |
5 |
3 |
8 |
|
5 |
1 |
1 |
2 |
2 |
1 |
3 |
0 |
1 |
1 |
1 |
2 |
3 |
1 |
1 |
2 |
0 |
0 |
0 |
|
6 |
6 |
4 |
10 |
1 |
4 |
5 |
1 |
1 |
2 |
1 |
3 |
4 |
0 |
1 |
1 |
1 |
0 |
1 |
|
?7 |
0 |
0 |
0 |
1 |
1 |
2 |
2 |
1 |
3 |
2 |
2 |
4 |
1 |
0 |
1 |
1 |
0 |
1 |
|
%age |
(0.3%) |
(26.98) |
(36.82) |
(23.17) |
(0.38) |
(0.76) |
||||||||||||
|
Age (Years) |
VACCINATED |
NON-VACCINATED |
||
|
|
MALE |
FEMALE |
MALE |
FEMALE |
|
1 |
1 |
0 |
0 |
1 |
|
2 |
30 |
30 |
13 |
11 |
|
3 |
50 |
26 |
21 |
16 |
|
4 |
25 |
23 |
13 |
8 |
|
5 |
4 |
3 |
1 |
3 |
|
6 |
7 |
9 |
3 |
4 |
|
7 |
3 |
3 |
4 |
1 |
|
Total |
214 |
99 |
||
References
- American Academy of Pediatrics, 2009:444-55. Isabel Peña-Rey1,2, *, MarÃÂa Victoria MartÃÂnez de Aragón1,2, Odorina Tello Anchuela1,2, Josefa Masa1, Enrique Alcalde Cabero1, MarÃÂa Teresa Castellanos Ruiz1 and Regional Coordinators of the MeaslesElimination Plan#1Servicio de Vigilancia Epidemiológica, Centro Nacional de EpidemiologÃÂa, Instituto de Salud Carlos III, 28028Madrid, Spain2CIBER Epidemiologia y Salud Publica (CIBERESP), Spain
- American Academy of Pediatrics. Measles. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL:
- Bernstein, D. I., Schiff, G.M., Measles. In: Infectious Diseases, Gorbach SL, Bartlett JG, Blacklow NR (Eds), WB Saunders, Philadelphia 1998. p.1296.
- Cherry, J. D., Measles, virus. In: Textbook of Pediatric Infectious Diseases, 6th ed, Feigin RD, Cherry JD, Demmler-Harrison GJ, et al (Eds), Saunders, Philadelphia 2009. p.2427.
- Epidemology, & Prevention. of vaccine preventable diseases, The Pink Book:Course Text Book-2th edition 2nd printing (may 2012) )Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189 Suppl 1: S4.BABBOTT FL Jr, GORDON JE. Modern measles. Am J Med Sci 1954; 228:334.
- Hopkins Bloomberg School of Public Health, Baltimore, Maryland
- Markowitz, L. E., Preblud, S. R., Fine, P. E., & Orenstein, W. A. (1990). Duration of live measles vaccine-induced immunity.Pediatr Infect Dis J. Feb 9(2),101-[Medline]
- mumps, & rubella-vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998;47(No. RR-8):1-57. (http://www.who.int/vaccines- documents/) http://emedicine.medscape.com/a rticle/966220-medication
- Robert, T., Perry, 1., & Neal, A. Halsey2 1National Immunization Program, Centers for Disease Control and Prevention, Atlanta, Georgia; 2Department of International Health, Johns
- Schneider-Schaulies, S., Schneider-Schaulies, J., & Measles. virus-induced immunosuppression. Curr Top MicrobiolImmunol. 2009; 330:243- 69. [Medline].
- Stephen, J., McPhee, Maxine, A., & Papadakis. (2011). Current Medical Diagnosis and Therapeutics,50th edition, McGraw- hillCompanies,USA.
- American Academy of Pediatrics, 2009:444-55. Isabel Peña-Rey1,2, *, MarÃÂa Victoria MartÃÂnez de Aragón1,2, Odorina Tello Anchuela1,2, Josefa Masa1, Enrique Alcalde Cabero1, MarÃÂa Teresa Castellanos Ruiz1 and Regional Coordinators of the MeaslesElimination Plan#1Servicio de Vigilancia Epidemiológica, Centro Nacional de EpidemiologÃÂa, Instituto de Salud Carlos III, 28028Madrid, Spain2CIBER Epidemiologia y Salud Publica (CIBERESP), Spain
- American Academy of Pediatrics. Measles. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL:
- Bernstein, D. I., Schiff, G.M., Measles. In: Infectious Diseases, Gorbach SL, Bartlett JG, Blacklow NR (Eds), WB Saunders, Philadelphia 1998. p.1296.
- Cherry, J. D., Measles, virus. In: Textbook of Pediatric Infectious Diseases, 6th ed, Feigin RD, Cherry JD, Demmler-Harrison GJ, et al (Eds), Saunders, Philadelphia 2009. p.2427.
- Epidemology, & Prevention. of vaccine preventable diseases, The Pink Book:Course Text Book-2th edition 2nd printing (may 2012) )Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004; 189 Suppl 1: S4.BABBOTT FL Jr, GORDON JE. Modern measles. Am J Med Sci 1954; 228:334.
- Hopkins Bloomberg School of Public Health, Baltimore, Maryland
- Markowitz, L. E., Preblud, S. R., Fine, P. E., & Orenstein, W. A. (1990). Duration of live measles vaccine-induced immunity.Pediatr Infect Dis J. Feb 9(2),101-[Medline]
- mumps, & rubella-vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1998;47(No. RR-8):1-57. (http://www.who.int/vaccines- documents/) http://emedicine.medscape.com/a rticle/966220-medication
- Robert, T., Perry, 1., & Neal, A. Halsey2 1National Immunization Program, Centers for Disease Control and Prevention, Atlanta, Georgia; 2Department of International Health, Johns
- Schneider-Schaulies, S., Schneider-Schaulies, J., & Measles. virus-induced immunosuppression. Curr Top MicrobiolImmunol. 2009; 330:243- 69. [Medline].
- Stephen, J., McPhee, Maxine, A., & Papadakis. (2011). Current Medical Diagnosis and Therapeutics,50th edition, McGraw- hillCompanies,USA.
Cite this article
-
APA : Shoukat, H., Pervaiz, F., & Noreen, S. (2017). Evaluation of Prevalence of Complications of Measles. Global Pharmaceutical Sciences Review, II(I), 34-41. https://doi.org/10.31703/gpsr.2017(II-I).04
-
CHICAGO : Shoukat, Hina, Fahad Pervaiz, and Sobia Noreen. 2017. "Evaluation of Prevalence of Complications of Measles." Global Pharmaceutical Sciences Review, II (I): 34-41 doi: 10.31703/gpsr.2017(II-I).04
-
HARVARD : SHOUKAT, H., PERVAIZ, F. & NOREEN, S. 2017. Evaluation of Prevalence of Complications of Measles. Global Pharmaceutical Sciences Review, II, 34-41.
-
MHRA : Shoukat, Hina, Fahad Pervaiz, and Sobia Noreen. 2017. "Evaluation of Prevalence of Complications of Measles." Global Pharmaceutical Sciences Review, II: 34-41
-
MLA : Shoukat, Hina, Fahad Pervaiz, and Sobia Noreen. "Evaluation of Prevalence of Complications of Measles." Global Pharmaceutical Sciences Review, II.I (2017): 34-41 Print.
-
OXFORD : Shoukat, Hina, Pervaiz, Fahad, and Noreen, Sobia (2017), "Evaluation of Prevalence of Complications of Measles", Global Pharmaceutical Sciences Review, II (I), 34-41
-
TURABIAN : Shoukat, Hina, Fahad Pervaiz, and Sobia Noreen. "Evaluation of Prevalence of Complications of Measles." Global Pharmaceutical Sciences Review II, no. I (2017): 34-41. https://doi.org/10.31703/gpsr.2017(II-I).04
