Abstrict
The study was aimed at developing a topical preparation of Celecoxib, a COX inhibitor available in oral dosage form only, for localized and systemic effects. Initially, celecoxib containing micro-emulsion was developed using liquid paraffin, tween 20, water, methyl paraben, propyl paraben, menthol and clove oil. These components were incorporated into Carbopol 934 gel, with varying ratio to achieve stable emulgel formulation. The emulgel appeared as smooth white homogenized formulation having pH 6.6-6.8. The rheological properties showed pseudo plastic nature with yield stress. The spreadability and extrudability showed sufficient consistency for ease of processing, filling and application. Microbial assay showed no contamination to ensure the stability of formulation upon storage. Ex-vivo bio-adhesive strength and skin irritation test showed no irritation on rat skin. All these parameters suggested that emulgel can be explored and developed as an effective alternative formulation for the local and systemic application of celecoxib. various body tissues, connected by blood circulatory system. Another name for non-compartmental analysis is model-independent approach that means it does not require any compartment model.
Keywords
Emulgel, Celecoxib, Carbopol 934, Topical
Introduction
Several physiological and pharmacological options are in practice to manage pain of different origins. When malfunctioning is handled effectively, the intensity of pain becomes less. Pain killers of different classes are usually used in the reduction of pain. Pain killers, also known as analgesics have best result in controlling the nociceptive pain but limited effect for neuropathic pain(Dworkin et al., 2007).Narcotics or opioid analgesics are the drugs having powerful pain-relieving potency particularly associated with surgeries or bone fractures, burns and other malignancies like cancer. The non-opioid analgesics are best to treat pain up to moderate intensity with the advantage of having no addition potential and they don’t show the condition in which the pain starts again after a short time. The major group of drugs includes NSAIDs which have extensively used globally for pain management. NSAIDs indicated for fever, mild to moderate pain, migraine, tension headache, bone cancer, rheumatoid arthritis, osteoarthritis and gout (Hebbes, 2016).
All types of NSAIDs work by blocking the prostaglandins secretion, the hormones, which is responsible for inducing inflammation, pain increased temperature and muscles cramps (Avouac, Combe, & Darne, 2003). Another important group of NSAIDs include COX (cyclooxygenase) inhibitors which can reduce pain and swelling in affected area and show less GIT problems. COX are present in two isoforms i.e. COX-1 (located in cells like endothelial, gastric mucosal, kidney cells and blood platelets) and COX-2 (overexpressed in conditions like inflammation or swelling of joints, skeletal muscles damage). NSAIDs can block both COX-1 and COX-2 selectively and non-selectively (Day & Graham, 2004). Celecoxib that is a COX-2 selective nonsteroidal anti-inflammatory drug (NSAID) and is used for treating the pain and swelling associated with osteoarthritis, rheumatoid arthritis. (Thangamani, Younis, & Seleem, 2015). Celecoxib inhibits prostaglandin synthesis by inhibiting COX-2 enzyme which produce prostaglandin from arachidonic acid. Then prostaglandins are transformed into active metabolites prostacyclin, thromboxane, prostaglandin D2, prostaglandin F2 that effect different physiological reaction that are inflammation, regulation of blood pressure and fever (Mathew, Devi S, Prasanth, & Vinod, 2011). Celecoxib is a reversible inhibitor of cyclooxygenase COX- 2; that inhibit the transformation of arachidonic acid into prostaglandin precursor. That’s why it has properties like antipyretic, anti-inflammatory and analgesic.
Solutions are mixture of water or alcoholic lotion with a dissolved powder. Topical solutions with low viscosity use alcohol as a base. Due to the presence of alcohol, dryness of the skin occurs. Solutions are made by adding water, alcohol or oil in the powder. Variety is seen in brands. (Singh Malik, Mital, & Kaur, 2016).Lotions are consisted of oil, water or alcohol. They are thicker than solution.. Transdermal patches are of specific dosing treatments includes the adhesives (Lee, Koo, & Wolverton, 2007). Creams are semisolid emulsion and melt when interact with skin. Preservatives and fragrances are included in it. Gels are thicker in nature as compare to liquids. Gels are semisolid emulsion. They use alcohol as a solvent for active ingredient. At body temperature Gels melt. Gels have stingy effect due to alcohol so on application; fissure should be avoided on skin. The dose of topical formulation can be increased when it is necessary. This type of medication helps in lessening the side effects along with the lessening the toxicity of organs. (Subacute & Chronic). Emulgel helps in avoiding the first pass effect, medication can be applied on its own to specific area. They also help to increase patient compliance. For drugs having short half-life and potent drugs, emulgel is favorable. Drug can be delivered to its specific site in emulgel form. (Joshi, Singh, Rana, Saini, & Singla, 2011)
The drugs having hydrophobic nature can’t enter into gel which acts as a hurdle, problem stands upon release. Emulgels assists intermixing of drugs of hydrophobic nature into oil part and these droplets of oil when distributed in aqueous part it results in the formation of oil/water type of emulsion. This emulsion then intermixes into gel phase. Which results into good stability. Noisome and liposomes are other novel approaches of Nano size, results in leakage because of vesicular structures and also show good entrapment effectiveness. Gels have good loading ability. (Joshi et al., 2011).Emulgels are good to be utilized as to increase the result of shorter T½ drug. Emulgels are less greasy and applied easily (Joshi et al., 2011).
Material and Method
Materials
Celecoxib received as gift from GETZ PHARMA PAKISTAN (PVT) LTD.), Propylene glycol (Sigma Aldrich made in Germany), Ethanol (Merck, Germany), Methyl paraben, (Sigma-Aldrich, Germany) Propyl paraben (Sigma-Aldrich, Germany), Clove oil (Sigma-Aldrich, Germany), Menthol (Sigma-Aldrich, Germany), Isopropyl Alcohol (Sigma-Aldrich, Germany), Carbopol 934, Benzyl Benzoate (Sigma-Aldrich, Germany), Diethanolamine (Sigma-Aldrich), Rose oil flavor. (Sigma-Aldrich, Germany), distilled water Liquid paraffin (Sigma Aldrich made in Germany), Tween 20 (Sigma Aldrich made in Germany), Span 20 (Sigma Aldrich made in Germany)
Methods
Synthesis of Emulgel
Schematic representation of Emulgel synthesis is shown in figure 1.
Emulsion Formation
The oily phase of emulsion was prepared by mixing Span 20 and liquid paraffin. To this, methyl paraben, propyl paraben, clove oil dissolved in ethanol were added. Tween 20 was mixed in water to formulate aqueous phase. Both solutions were heated at 70-80 Cº separately. Oily phase added to aqueous phase with constant stirring to develop stabilized emulsion.
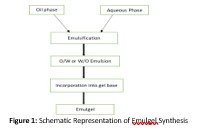
Preparation of Gel
Transferred 150ml purified water to the compounding vessel (beaker). Added Carbopol 934 gradually and mixed well to homogenize until the solution becomes lump free. Heated isopropyl alcohol to a temperature of 59-63Cº in a beaker, then added celecoxib in it, mixed well until clear solution is obtained. Then added benzyl benzoate in it. Transferred it to step1 with slow continuous agitation. Added and mixed Methyl paraben sodium, Propyl paraben sodium and Diethanolamine in 240ml purified water for 10minutes. Continue mixing at fast rate till it become clear. Rose oil added for fragrant purpose. After continuous mixing for 30 minutes added rose oil drops in it and mix for 20minutes. Various compositions of
celecoxib emulgel were formed as shown in table 1.
Table 1: Composition of Celecoxib Emulgel (Various Formulations)
|
Ingredients |
Formulation 1(F1) |
Formulation 2(F2) |
Formulation 3(F3) |
Formulation 4(F4) |
|
Celecoxib |
500 |
500 |
500 |
500 |
|
Isopropyl Alcohol |
32 |
32 |
32 |
32 |
|
Carbopol 934P |
1.5 |
2 |
2.7 |
3.5 |
|
Benzyl Benzoate |
2 |
2 |
2 |
2 |
|
Diethanolamine |
2.4 |
2.4 |
2.4 |
2.4 |
|
Propyl Paraben Sodium |
0.1 |
0.1 |
0.1 |
0.1 |
|
Rose oil flavor |
0.4 |
0.4 |
0.4 |
0.4 |
|
Liquid Paraffin |
7.5 |
7.5 |
7.5 |
7.5 |
|
Tween 20 |
0.5 |
0.5 |
0.5 |
0.5 |
|
Span 20 |
1 |
1 |
1 |
1 |
|
Propylene Glycol |
5 |
5 |
5 |
5 |
|
Ethanol |
2.5 |
2.5 |
2.5 |
2.5 |
|
Methyl Paraben |
0.03 |
0.03 |
0.03 |
0.03 |
|
Ethyl Paraben |
0.01 |
0.01 |
0.01 |
0.01 |
|
Clove oil |
0.4 |
0.4 |
0.4 |
0.4 |
|
Menthol |
4 |
4 |
4 |
4 |
|
Purified Water |
176 |
176 |
176 |
176 |
Characterization
Physical appearance
Emulsion formulation will be scrutinized visually for color, homogeneity, consistency and ph. The pH values of aqueous solution of jellified emulsion will be measured by pH meter. (Khullar, Kumar, Seth, & Saini, 2012).
Determination of pH
Digital pH meter was used to determine pH that was calibrated with standard buffer solution. The measurements were replicated thrice. (Vinod & Vani).
Rheological study
Different emulgel formulations viscosity was be determined at 25ºC by using a cone and plate viscometer and that will be connected to thermostatically controlled water bath. (Joshi et al., 2011).
Viscosity
The viscosity of formulations prepared, was determined at ambient temperature by using digital viscometer. (Basera, Kothiyal, & Gupta, 2015).
Swelling index
Swelling index of prepared emulgel will be determined by taking 1gm of gel on porous aluminum foil and then it would be placed separately in a 50ml beaker containing 10ml of 0.1N NaOH. Then the sample would be
removed from beaker different intervals and it will be placed to dry for some time later it will be reweighed.
Swelling index (SW)%= [(Wt –Wo) /Wo ] x100
SW % = equilibrium percentage swelling
Wo = original weight of the emulgel at zero time after time t
Wt. = weight of the swollen emulgel (Joshi et al., 2011).
Spreadibility
Spreadibility was determined by apparatus which will be suitably modified in the laboratory and used for the study. It will consist of a wooden block, which will be provided by pulley at one end. Spreadibility measured on the basis of ‘slip’ and ‘drag’ characteristics of emulgels. A ground glass slide will be fixed on block. An excess of emugel was placed on this ground slide. The emulgel then was sandwiched between this slide and another glass slide. A 1 Kg weight placed on the top of two slides for 5 minutes to expel air and to provide uniform film of the emulgel among slides. Then the excessive emulgel was scraped off from the edges. Shorter interval was indicating Spreadibility. Spreadibility was calculated by this formula,
S=M.L/T
S= Spreadibility
M= weight tied to upper slide
L= length of glass slides
T= time taken to separate the slides completely from each other (Vinod & Vani)
Extrudability Study
It was empirical test used to determine the strength that is essential to expel the material out from tube. This technique was adopted for analyzing emulgel formulation for Extrudability depending upon quantity in percentage of emulgel and emulgel extruded from lacquered aluminum collapsible tube when applied weight in grams required to extrude minimally 0.5cm ribbon of emulgel in 10seconds. (Singla, Saini, Joshi, & Rana, 2012).
Extrudability= applied weight to extrude Emulgel from tube (in grams) / Area (in cm)
Ex-vivo bio–Adhesive Strength Measurement
Shaven skin of mice was used to measure ex-vivo bio adhesive strength. The fresh skin of mice was cut into pieces, and it was washed with 0.1N NaOH. Out of them two pieces of skin were tied to two glass slides, out of them one glass slide was fixed on wooden box and other was tied to the balance. The right and left pans were balanced by adding extra weight. 1gm of Celecoxib Emulgel was placed between these slides having shaven skin of mice and extra weight was removed to remove air. The balance was kept in that position for 5minute. To left hand pan weight was added slowly until patch detached from skin. (Joshi et al., 2011).
The formula used to calculate bio-adhesive strength was as:
Bio adhesive strength = Weight required (in gm) / Area (cm2)
Drug Content Determination
Spectrophotometer used to measure drug concentration jellified emulsion. It was measured by dissolving known quantity of jellified emulsion in solvent by sonication. Absorbance was measured after suitable dilution in UV/Vis spectrophotometer. (Joshi et al., 2011).
Stability Studies
The prepared emulgel was packed in aluminum collapsible tubes and was subjected to stability studies at 5 C, 25 C / 60% RH, 30 C /65 RH, and 40C / 75 RH for about 3 months’ period. Samples was withdrawn at 15day time intervals and physical appearance, pH, rheological properties, drug content and drug release content evaluated. (Mohamed, 2004).
Skin Irritation Test
Sample of the test material of about 0.5gm was applied to each side (two sites per rabbit) by introducing under a double gauze layer to an area of skin. After 24-hour emulgel was removed. There was no irritation occurred that is why the test was passed. (Singla et al., 2012).
Results
Physical Examination of Emulgel
The Celecoxib Emulgel formulations were examined visually for their color, homogeneity, consistency and phase separation as shown in figure 2.

Figure 2
Figure 2: (a) Physical appearance of celecoxib Emulgel, (b) consistency and
homogeneity of Celecoxib Emulgel
pH Determination
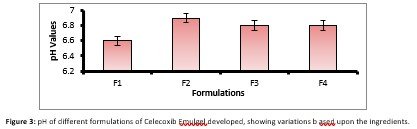
Digital pH meter was used to determine the pH of Celecoxib Emulgel that was calibrated with solutions of standard buffer. pH measurements of each preparation were repeated thrice. pH was found to be 6.6-6.8 as shown in figure 3.
Rheological Study
The viscosity of the Celecoxib Emulgels was determined separately using viscometer with spindle 4 and rpm 6 that was connected to thermostatically controlled circulating water bath and results were shown in figure 4.
The formulation of Emulgel using Carbopol 934 as gelling agent is more effective in thick formulation such as emulsions, transdermal and topical.

Determination of Viscosity
The viscosity of different prepared formulations was analyzed at ambient temperature by using Brookfield digital viscometer. For topical use rheological properties of pharmaceutical system effect the rate of release of active substances present in it. Viscosity of gel was measured in kept constant.

Swelling Index
Swelling index of the topical Celecoxib Emulgel
prepared was determined at different intervals shown in table 2.
Table 2. Swelling Index
|
S.no |
Formulation |
Swelling Index percentage at
different time intervals |
||
|
30
min |
60
min |
90min |
||
|
1 |
F1 |
19.02% |
6.87% |
.68% |
|
2 |
F2 |
21.22% |
8.09% |
.87% |
|
3 |
F3 |
22.33% |
9.629% |
.95% |
|
4 |
F4 |
25.34% |
11.37% |
1.06% |
Spread ability
When topical therapy used its efficacy depends upon the
factor that how evenly the formulation is spreaded that it delivers the
standard dose in patients. This is specifically important with the formulations
of potent drugs. Spreadability of celecoxib emulgel is shown in figure 6.

Extrudability Study Emulgel Topical
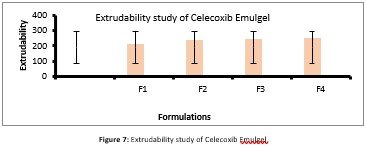
It is regular factual test that was performed to determine the force needed to extrude Emulgel from the tube. This technique was applied for measurement of applied force in the rheogram region comparable to rate of shear that exceeds the value of yield and showed related plug flow. Currently, this technique ratifies for analyzing preparation of emulgel for Extrudability that was based upon the quantity of emulgel percentage and when weight in grams was applied on the aluminum collapsible tube emulgel extruded out at least 0.5cm of celecoxib emulgel in 10seconds and results were shown in figure 7. If more quantity extrudes out, then it shows better Extrudability.
Ex vivo Bio Adhesive Measurement of Strength of Celecoxib Emulgel Topical
The improved technique was used for determining the bio adhesive strength. The fresh skin of mice was cut into pieces and washed with 0.1 N NaOH. Two pieces were picked and tied to the two slides of glass separately out of which one slide was fixed on the piece of wooden and other piece of skin was tied with the balance on right hand side. Emulgel pressure was applied on it to remove air. The balance was kept in this same position for 5minutes. Weight was added to it slowly at 200mg /min to the pan on left hand until patch was detached from the surface of skin. (Joshi baibhav2011). The weight that was needed to detach/separate the Celecoxib Emulgel from the surface of skin gave determination of bio-adhesive strength and results were shown in figure 8

Solubility Studies of Celecoxib
Solubility of Celecoxib in different medias was observed by dissolving it in 0.1N Hcl, acetate buffer, phosphate buffer and purified water and resukts were shown in table 3.
Table 3: Solubility Studies of Celecoxib Emulgel in Different Media
|
S.no |
Media |
Solubility in mg/ml |
Solubility in mg/250ml |
|
1 |
0.1 N Hcl |
0.0272 |
6.8 |
|
2 |
pH 4.5 Acetate Buffer |
0.0252 |
6.3 |
|
3 |
pH 6.8 Phosphate Buffer |
0.015 |
3.8 |
|
4 |
pH 7.4 Phosphate Buffer |
0.0148 |
3.7 |
|
5 |
pH 12 tribasic sodium phosphate
buffer |
0.572 |
143 |
|
6 |
Purified water |
0.0204 |
5.1 |
UV- Analysis
Ultraviolet visible spectroscopy
is also known as absorption spectroscopy that means it uses light in visible
ranges. Absorbance and concentration of Celecoxib emulgel was determined.
Dilutions were made in methanol and maximum wavelength observed on 254nm.
Standard curve of celecoxib is shown in figure 10.

Microbiological Assay
Petri dishes were used for this technique. This process was used for analysis of fungistatic or bacteriostatic action of compound. Majorly this formulation was applied for semisolid preparations. Agar medium was prepared and dried in the petri dishes. Emulgel was applied in the form of streaks on the agar medium in petri dishes and kept for incubation. After incubation of 24 hours at 25 C°. (Joshi baibhav.2011) No fungal or bacterial growth was observed on the petri dishes.

Figure 11: (a) Agar medium prepared and dried in petri dishes (b) Celecoxib Emulgel was applied on petri dishes (c) After incubation no bacterial or fungal growth shown on petri dishes
Accelerated Stability Studies
The Celecoxib Emulgel formulations were stored at two different chambers for the period of 3 months. The samples were analyzed for change in pH of Emulgel and physical appearance at regular intervals of time. (Joshi
baibhav.2011) The specifications of the chambers were as follows
• Chamber 1 (65% humidity and 30 C°)
• Chamber 2(75% humidity and 40 C°)
No change in pH and physical appearance was observed after the completion of the specified time of 3 months.
Skin Irritation Test (Patch Test)
The Celecoxib Emulgel was applied on the shaven skin of rat properly and its adverse such as color change, skin morphology changes were checked up to 24hour. No irritation was seen in rats during the study.
Figure 11: (a) Agar medium prepared and dried in petri dishes (b) Celecoxib Emulgel was applied on petri dishes (c) After incubation no bacterial or fungal growth shown on petri dishes
Accelerated Stability Studies
The Celecoxib Emulgel formulations were stored at two different chambers for the period of 3 months. The samples were analyzed for change in pH of Emulgel and physical appearance at regular intervals of time. (Joshi
baibhav.2011) The specifications of the chambers were as follows
• Chamber 1 (65% humidity and 30 C°)
• Chamber 2(75% humidity and 40 C°)
No change in pH and physical appearance was observed after the completion of the specified time of 3 months.
Skin Irritation Test (Patch Test)
The Celecoxib Emulgel was applied on the shaven skin of rat properly and its adverse such as color change, skin morphology changes were checked up to 24hour. No irritation was seen in rats during the study.

Figure 13: Application of Celecoxib Emulgel on rat skin (a) shaven skin of rat (b)
Celecoxib emulgel applied on the shaven skin of rat (c) after 24hours rat skin
with no rashes and irritation
Discussion
Emulgels are formed by combining gels and emulsions. When these are used for dermatological purpose, they have some good properties such as greaseless, easily spreadable, thixotropic, easily removable, emollient, non-staining, longer shelf life, soluble in water, bio friendly and pleasing appearance. Emulgels are preferable as they are easily spreadable and are conveniently applied on skin. When the drug is applied to transdermal, it has advantages that it avoids harms related with intravenous therapy and difficulties related with different gastric pH and time required for emptying. It has aqueous and non-aqueous phases that shows ability to deliver hydrophilic and lipophilic agents. Hence, emulgels are most stable thermodynamically when it is compared to emulsions. The present research was aimed to develop celecoxib emulgel comprising of liquid paraffin, span 20, methyl paraben, propyl paraben, clove oil and ethanol as component of emulsion. Gel was prepared using Carbopol 934 and was mixed well to homogenize until the solution becomes lump free. The drug loaded emulgel was successfully prepared by mixing emulsion and gel preparations.
Celecoxib emulgel was assessed for its macroscopic attribute such as color, pH viscosity, swelling index, skin irritation test. Celecoxib emulgel showed smooth texture, milky white color and was homogeneous with good consistency. Stability of semisolids is affected by the change in particle size, polymorphic or hydration state.
Consistency and caking/calescence also changes stability of semisolid preparations. If stability is disturbed then drug content is lost, that results in change in release rate of drug and loss of elegance.
Rate of drug decomposition is effected by acidic and alkaline pH. Many drugs are stable between pH range of 4-8. Good solubility is observed in weekly acidic and basic drugs because they decompose faster in their ionized form. pH also effects decomposition of the drug, as pH change of 1 unit can result in 10folds change. (Palacio & Bhushan, 2012). A change in pH can cause irritation and skin disruption. If the pH value of the emulgel applied to the skin is acidic in nature or alkaline, it will results in skin irritation/ vexation. According to the studies, the best range of solution for skin application is between 6.8 to 7.3 (Khullar et al., 2012). The pH value of all developed formulations of Celecoxib Emulgel (F1, F2, F3, F4) was 6.8, which is best suitable to the skin applications.
Thickness of the fluid is known as viscosity; it is interaction between different molecules. Temperature and shear rate have obvious effects on the viscosity, some substances are mostly temperature sensitive and minor change in temperature results in major change in their viscosity (Maheshwar). Semisolid materials have both liquid and solid properties within one product that’s why they are most difficult to characterize rheologically (Islam, Rodriguez-Hornedo, Ciotti, & Ackermann, 2004). Drug delivery of semisolid dosage forms can be influenced by its viscosity. Diffusion rate of drug may also be effected by viscosity of the product. Hence behavior of flow must be observed after application of medicine. (Kadhim, Seah, & Zubair, 2016) Correct formulation of ingredients makes gels and emulgels easily flow out of the container that assures, while storing preparation no sedimentation of solid particles occurs and it is stable and have excellent spreadability on the surface. When the shear stress is increased the disarranged molecules of the gelling agent arrange themselves in the direction of flow. Because of this type of orientation/arrangement decreases the internal resistance and also decreases viscosity of the material (Panwar et al., 2011). When the concentration of Carbopol 940 and Carbopol 934 increases then the viscosity of emulgel also increases. Clove oil and emulsifier concentrations also affects the rheological properties of Emulgel. Viscosity of the preparation plays much important role in the complete extrusion of the formulation from primary packing, while applying it is properly handled, and in primary packing stabilization of the dispersed phase and homogeneous distribution of the drug in package is maintained during its shelf life. Emulgel is formulated by using Carbopol 934 and Carbopol 941 separately, and their viscosities observed. Formulation made from Carbopol 934 showed better thickness and viscosity for topical preparation as compared to Carbopol 941. The pH value of Celecoxib emulgel was found to be in range 6.8 to 7.3 with the carbopol to obtain good viscosity.
Intrinsic mechanism that leads to thixotropic behavior is based on three dimensional structure, created by the molecules that are capable secondary bond interactions, during shear that are broken, decrease in viscosity, then gel turns into solution form shown in ascending curve. When the shearing stress is removed, recovery process is slow initial structure, necessary time for restructuring required by grouping molecules, that are in Brownian motion, which depend on the applied shear stress. Degradation of semisolid structure was shown by thixotropic behavior when the shear stress was applied. By analyzing the rheograms, we can state that after certain period of rest the gel returns to its initial viscosity depending upon the structure of molecule. It is necessary to analyze Thixotropic behavior of gels for topical semisolid systems (when product applied on skin initially its thick then under stress it becomes thinner and easily spreadable).
Swelling is a usual phenomenon of interaction of a polymer with solvent. Insertion of such liquid within polymer matrix tends to swell it. This could be either reversible or irreversible. Many times, some polymer has a tendency to absorb moisture which tends to change the associated properties. The total moisture insertion is not always reversible. A certain fraction forms a part the polymer matrix. Simple way to calculate the percent swelling is to consider the difference of weight of the polymer before and after. (Kadhim et al., 2016) Swelling index for different formulations prepared was observed at different time intervals that is after 30min it was 19.02%, 21.22%. 22.33%, 25.34% for formulations F1,F2,F3,F4 respectively.
Spreadability of a formulation exhibits the area on which gel readily spreads when it is applied on the skin. Spreadability is one of the most important criteria for emulgel. It is dependent upon the type and concentration of polymer that are used to make formulation.(Garg, Aggarwal, Garg, & Singla, 2002) Formulations that are more viscous shows low spreadability and vice versa. The value of spreadability shows that by small amount of shear the emulgel is easily spreaded on the site of application. The spreadability is most important because it also shows the behavior of emulgel comes out of the container. (Aiyalu, Govindarjan, & Ramasamy, 2016) When the concentration of any gelling agent is increased then the spreadability of preparation decreases. The spreadability of Celecoxib emulgel was considered high by having low spread of time. The therapeutic efficiency of emulgels depends on their spread. Moreover, this is the main aspect in compliance of patients with treatment. Formulation F1 have showed more spreading coefficient i,e. 177.7gcm/sec. when it is compared to other formulations, the reason is that it contains optimum concentration of Carbopol 934. Other formulations such as F2, F3 and F4 have values 128, 60.95, 41.63 respectively. The reason for decreasing spreadability value is formulation becomes more concentrated as gelling agent concentration is increased that is why it shows low spreadability value and covers lesser distance.
The consistency shows the capacity of the emulgel to get ejected in uniform manner and desired quantity when the tube is squeezed. The consistency of the material is major feature in analgesic and anti-inflammatory topical preparations because it is applied to the skin having thin layers, hence the drug permeation is controlled by viscosity of the emulgel. The consistency of my emulgel formulation was good as it was ejected from tube in uniform manner and gave good results.
Extrudability is the force required to push something out. A compression-extrusion test is to apply force to a product until it comes out of container that can be one or more than one holes in the test cell. The product is compressed until its structure disrupts and product extrudes out of these holes.in case of semisolids it is important to determine quantifying extrudability, to observe its ease of removal and its application (Kumar & Verma, 2010). Any changes in consistency is observed during its shelf life, it also helps to access which packaging material is suitable for formulation. Celecoxib Emulgel showed extrudability in range of 214- 253g that was much close to clotrimazole gel (190g) available in market. From this data it is concluded that emulgel prepared from hydroxymethyl cellulose have less drug content, extrudability, spreadability and bio adhesive strength as compared to prepared with natural polymer.
In pharmaceutical development important component is the stability studies, in this stability of active pharmaceutical ingredient is evaluated and stability of drug product under effect of environmental changes like temperature, humidity, and light (Kumar & Verma, 2011). These studies enable to make recommendations for storage conditions and shelf-life. The celecoxib emulgel was exposed to stress conditions under stability testing. During the stability studies the formulation appeared clear and there was no pH variation. The prepared Celecoxib emulgel were found to be stable upon storage for 6 months at room temperature, no significant change was observed in parameters like color, pH, rheological properties, drug release and fungistatic and bacteriostatic properties.
Bio-adhesion is the ability of material to stick to a tissue for an extended time period. It is effected by various factors like molecular weight of the polymer, polymer concentration, flexibility of polymer chains, polymer pH, swelling, initial time contact and the strength applied. Polymer used for bio-adhesion should have characteristics of flexibility that means interpenetration between polymers and mucosal membranes, it should be hydrophilic in nature, forms strong adhesion bonds, have high molecular weight (Palacio & Bhushan, 2012). Bio adhesive strength for celecoxib formulation was observed 2.5g/cm2. Carbopol 934 is an excellent polymer for bio-adhesion that is use in Celecoxib Emulgel formulation. Bio-adhesion bond formation process occurs in three steps: wetting and swelling of polymer, mucosal membrane and polymer chain interpenetration, chemical bond formation between the entangled chains. Bio-adhesive strength of Celecoxib Emulgel was dependent on gelling agent concentration and Carbopol. The characteristics such as polymer chain flexibility, hydrogen bond forming ability, and polymer swelling extent effect bio-adhesive strength of developed formulation.
Antimicrobial assay was performed on celecoxib emulgel to analyze bacteriostatic and fungistatic action. Agar medium was prepared and emulgel was applied on it and kept for 24hours at 25C ?. No bacterial or fungal growth was shown.
When the chemicals are topically applied then it can result in adverse effects.by severity and reversibility of effects one can differentiate skin burns from skin irritation. Substances that are corrosive in nature irreversibly damage skin that cannot be repaired while the substances that are irritant to the skin lead to reversible local reaction causing inflammation that can cause tissue trauma or cell damage, in this condition skin cells release inflammatory mediators that enhance the permeability and diameter of vessels, immune cells attract towards the injury site that trigger the immune cells by endothelium by tissue, then they clear antigen and repair tissue. Furthermore, mediators of inflammation trigger endings of the nerves that leads to itching and sensation of stinging. Recently test methods that are accepted internationally for skin irritation testing are in vivo animal test as well as in vitro test methods. The skin irritation studies for Celecoxib Emulgel was done on rat that confirmed no irritation no change in color or morphology of skin of shaved rat.
Conclusion
Celecoxib can be delivered topically as Emulgel in the treatment of relieving pain of osteoarthritis, rheumatoid arthritis, ankylosing spondylitis to manage acute pain and primary dysmenorrheal pain as it has been observed that Celecoxib Emulgel formulations have good consistency, homogeneity, stability and spreadability and remained unchanged upon storage of 3 months. Incorporating Celecoxib into gel base increases the ease of its application onto skin. Till date, Celecoxib is not commercially available due to its hydrophobic nature it is difficult to formulate it in gel. Amongst all formulations of Emulgel prepared with Carbopol 934P was better with respect to overall product qualities. Emulsion system gives solublization of hydrophobic drug, hence drug availability in the formulation is increased. The Emulgel formulation showed no irritation when applied on the rat skin. Drug delivery applied topically have wider prospects. Thus, results of current study show that Celecoxib Emulgel can be a good substitute/alternative to conventional dosage form. Moreover, proposed concept of Celecoxib Emulgel can be made available to common man after its technology transferred to pharmaceutical industries.
References
- Aiyalu, R., Govindarjan, A., & Ramasamy, A. (2016). Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Brazilian Journal of Pharmaceutical Sciences, 52(3), 493-507.
- Avouac, B., Combe, B., & Darne, B. (2003). Prescription of NSAIDs in patients treatment with platelet inhibitors or anticoagulants. Presse medicale (Paris, France: 1983), 32(37 Pt 2), S38-43.
- Basera, K., Kothiyal, P., & Gupta, P. (2015). Nanoemulgel: a novel formulation approach for topical delivery of hydrophobic drugs. World Journal of Pharmacy and Pharmaceutical Sciences, 4(10), 1871-1886.
- Day, R. O., & Graham, G. G. (2004). The vascular effects of COX-2 selective inhibitors. Australian Prescriber, 27(6), 142-145.
- Dworkin, R. H., O'connor, A. B., Backonja, M., Farrar, J. T., Finnerup, N. B., Jensen, T. S., . . . Nurmikko, T. J. (2007). Pharmacologic management of neuropathic pain: evidence- based recommendations. Pain, 132(3), 237- 251.
- Garg, A., Aggarwal, D., Garg, S., & Singla, A. K. (2002). Spreading of semisolid formulations: an update. Pharmaceutical Technology North America, 26(9), 84-84.
- Hebbes, C. (2016). Non-opioid analgesics. Anaesthesia & Intensive Care Medicine, 17(9), 469-472.
- Islam, M. T., Rodriguez-Hornedo, N., Ciotti, S., & Ackermann, C. (2004). Rheological characterization of topical carbomer gels neutralized to different pH. Pharmaceutical research, 21(7), 1192-1199.
- Joshi, B., Singh, G., Rana, A., Saini, S., & Singla, V. (2011). Emulgel: a comprehensive review on the recent advances in topical drug delivery. Int Res J Pharm, 2(11), 66-70.
- Kadhim, A. A., Seah, B. T., & Zubair, A. M. (2016). Influence of magnetic field on blood viscosity. Advances in Environmental Biology, 10(1), 107- 111.
- Khullar, R., Kumar, D., Seth, N., & Saini, S. (2012). Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi pharmaceutical journal, 20(1), 63-67.
- Kumar, L., & Verma, R. (2010). In vitro evaluation of topical gel prepared using natural polymer. International journal of drug delivery, 2(1).
- Kumar, L., & Verma, R. (2011). Chemical stability studies of bioadhesive topical gel. International Journal Pharmacy and Pharmaceutical Scinces, 3(1), 101-104.
- Lee, C., Koo, J., & Wolverton, S. (2007). Comprehensive dermatologic drug therapy. Comprehensive dermatologic drug therapy, 11.
- Maheshwar, M. A REVIEW ARTICLE ON MEASUREMENT OF VISCOSITY.
- Mathew, S. T., Devi S, G., Prasanth, V., & Vinod, B. (2011). Efficacy and safety of COX-2 inhibitors in the clinical management of arthritis: Mini review. ISRN pharmacology, 2011.
- Mohamed, M. I. (2004). Optimization of chlorphenesin emulgel formulation. The AAPS journal, 6(3), 81-87.
- Palacio, M. L., & Bhushan, B. (2012). Bioadhesion: a review of concepts and applications. Phil. Trans. R. Soc. A, 370(1967), 2321-2347.
- Panwar, A., Upadhyay, N., Bairagi, M., Gujar, S., Darwhekar, G., & Jain, D. (2011). Emulgel: a review. Asian J Pharm Life Sci, 2231, 4423.
- Singh Malik, D., Mital, N., & Kaur, G. (2016). Topical drug delivery systems: a patent review. Expert opinion on therapeutic patents, 26(2), 213-228.
- Singla, V., Saini, S., Joshi, B., & Rana, A. (2012). Emulgel: A new platform for topical drug delivery. International Journal of Pharma and Bio Sciences, 3(1), 485-498.
- Subacute, C., & Chronic, C. DermNet NZ
- Thangamani, S., Younis, W., & Seleem, M. N. (2015). Repurposing celecoxib as a topical antimicrobial agent. Frontiers in microbiology, 6, 750.
- Vinod, V., & Vani, C. S. V. Overview on transdermal drug delivery by semisolid systems: Emulgel.
- Aiyalu, R., Govindarjan, A., & Ramasamy, A. (2016). Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Brazilian Journal of Pharmaceutical Sciences, 52(3), 493-507.
- Avouac, B., Combe, B., & Darne, B. (2003). Prescription of NSAIDs in patients treatment with platelet inhibitors or anticoagulants. Presse medicale (Paris, France: 1983), 32(37 Pt 2), S38-43.
- Basera, K., Kothiyal, P., & Gupta, P. (2015). Nanoemulgel: a novel formulation approach for topical delivery of hydrophobic drugs. World Journal of Pharmacy and Pharmaceutical Sciences, 4(10), 1871-1886.
- Day, R. O., & Graham, G. G. (2004). The vascular effects of COX-2 selective inhibitors. Australian Prescriber, 27(6), 142-145.
- Dworkin, R. H., O'connor, A. B., Backonja, M., Farrar, J. T., Finnerup, N. B., Jensen, T. S., . . . Nurmikko, T. J. (2007). Pharmacologic management of neuropathic pain: evidence- based recommendations. Pain, 132(3), 237- 251.
- Garg, A., Aggarwal, D., Garg, S., & Singla, A. K. (2002). Spreading of semisolid formulations: an update. Pharmaceutical Technology North America, 26(9), 84-84.
- Hebbes, C. (2016). Non-opioid analgesics. Anaesthesia & Intensive Care Medicine, 17(9), 469-472.
- Islam, M. T., Rodriguez-Hornedo, N., Ciotti, S., & Ackermann, C. (2004). Rheological characterization of topical carbomer gels neutralized to different pH. Pharmaceutical research, 21(7), 1192-1199.
- Joshi, B., Singh, G., Rana, A., Saini, S., & Singla, V. (2011). Emulgel: a comprehensive review on the recent advances in topical drug delivery. Int Res J Pharm, 2(11), 66-70.
- Kadhim, A. A., Seah, B. T., & Zubair, A. M. (2016). Influence of magnetic field on blood viscosity. Advances in Environmental Biology, 10(1), 107- 111.
- Khullar, R., Kumar, D., Seth, N., & Saini, S. (2012). Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi pharmaceutical journal, 20(1), 63-67.
- Kumar, L., & Verma, R. (2010). In vitro evaluation of topical gel prepared using natural polymer. International journal of drug delivery, 2(1).
- Kumar, L., & Verma, R. (2011). Chemical stability studies of bioadhesive topical gel. International Journal Pharmacy and Pharmaceutical Scinces, 3(1), 101-104.
- Lee, C., Koo, J., & Wolverton, S. (2007). Comprehensive dermatologic drug therapy. Comprehensive dermatologic drug therapy, 11.
- Maheshwar, M. A REVIEW ARTICLE ON MEASUREMENT OF VISCOSITY.
- Mathew, S. T., Devi S, G., Prasanth, V., & Vinod, B. (2011). Efficacy and safety of COX-2 inhibitors in the clinical management of arthritis: Mini review. ISRN pharmacology, 2011.
- Mohamed, M. I. (2004). Optimization of chlorphenesin emulgel formulation. The AAPS journal, 6(3), 81-87.
- Palacio, M. L., & Bhushan, B. (2012). Bioadhesion: a review of concepts and applications. Phil. Trans. R. Soc. A, 370(1967), 2321-2347.
- Panwar, A., Upadhyay, N., Bairagi, M., Gujar, S., Darwhekar, G., & Jain, D. (2011). Emulgel: a review. Asian J Pharm Life Sci, 2231, 4423.
- Singh Malik, D., Mital, N., & Kaur, G. (2016). Topical drug delivery systems: a patent review. Expert opinion on therapeutic patents, 26(2), 213-228.
- Singla, V., Saini, S., Joshi, B., & Rana, A. (2012). Emulgel: A new platform for topical drug delivery. International Journal of Pharma and Bio Sciences, 3(1), 485-498.
- Subacute, C., & Chronic, C. DermNet NZ
- Thangamani, S., Younis, W., & Seleem, M. N. (2015). Repurposing celecoxib as a topical antimicrobial agent. Frontiers in microbiology, 6, 750.
- Vinod, V., & Vani, C. S. V. Overview on transdermal drug delivery by semisolid systems: Emulgel.
Cite this article
-
APA : Hasan, Z., Anwar, M., & Sohail, M. F. (2016). Synthesis and In-vitro Characterization of Celecoxib Emulgel. Global Pharmaceutical Sciences Review, I(I), 17-28. https://doi.org/10.31703/gpsr.2016(I-I).03
-
CHICAGO : Hasan, Zahrah, Maryam Anwar, and Muhammad Farhan Sohail. 2016. "Synthesis and In-vitro Characterization of Celecoxib Emulgel." Global Pharmaceutical Sciences Review, I (I): 17-28 doi: 10.31703/gpsr.2016(I-I).03
-
HARVARD : HASAN, Z., ANWAR, M. & SOHAIL, M. F. 2016. Synthesis and In-vitro Characterization of Celecoxib Emulgel. Global Pharmaceutical Sciences Review, I, 17-28.
-
MHRA : Hasan, Zahrah, Maryam Anwar, and Muhammad Farhan Sohail. 2016. "Synthesis and In-vitro Characterization of Celecoxib Emulgel." Global Pharmaceutical Sciences Review, I: 17-28
-
MLA : Hasan, Zahrah, Maryam Anwar, and Muhammad Farhan Sohail. "Synthesis and In-vitro Characterization of Celecoxib Emulgel." Global Pharmaceutical Sciences Review, I.I (2016): 17-28 Print.
-
OXFORD : Hasan, Zahrah, Anwar, Maryam, and Sohail, Muhammad Farhan (2016), "Synthesis and In-vitro Characterization of Celecoxib Emulgel", Global Pharmaceutical Sciences Review, I (I), 17-28
-
TURABIAN : Hasan, Zahrah, Maryam Anwar, and Muhammad Farhan Sohail. "Synthesis and In-vitro Characterization of Celecoxib Emulgel." Global Pharmaceutical Sciences Review I, no. I (2016): 17-28. https://doi.org/10.31703/gpsr.2016(I-I).03
